by Brian Shilhavy
Editor, Health Impact News
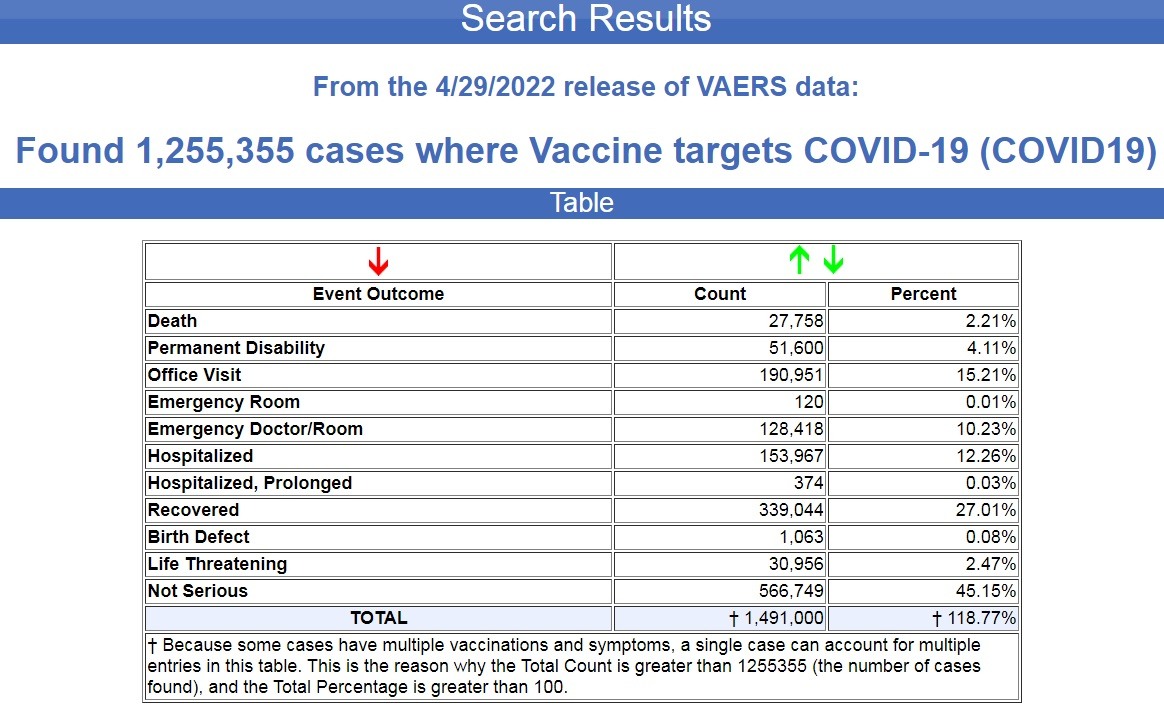
The U.S. Government Vaccine Adverse Events Reporting System (VAERS) was updated today, and there have now been 1,255,355 cases of adverse reactions filed following COVID-19 vaccines since December of 2020, a 17-month time frame.
This includes 27,758 deaths and 51,600 permanent disabilities. (Source.)
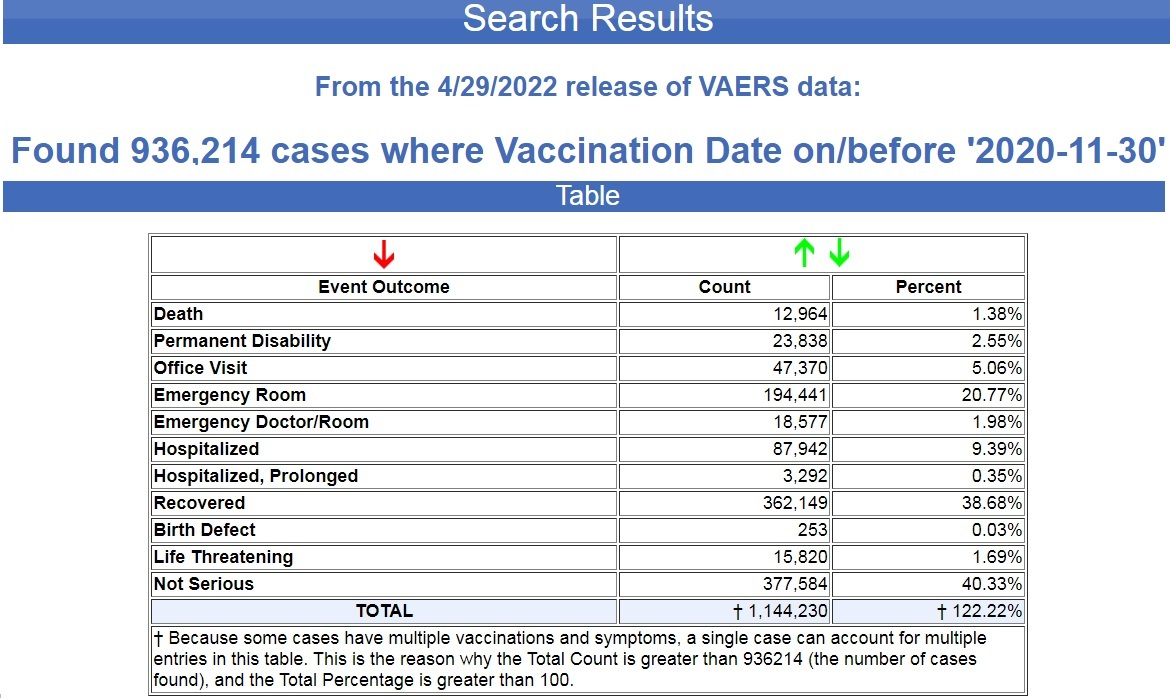
By way of contrast, for the previous 30 years before the COVID vaccines were rushed to market with EUAs (emergency use authorizations), there were 936,214 cases reported with 12,964 deaths and 23,838 permanent disabilities following all FDA-approved vaccines during a 360-month period. (Source.)
That’s a 4,434.22% increase in deaths following COVID-19 vaccines, compared to deaths following ALL FDA-approved vaccines for the previous 30 years.
And yet, the CDC continues to refer to COVID-19 vaccines as “safe and effective.”
“Effective” in what? The symptoms associated with “COVID-19” are easily treatable as many doctors have said since the beginning of the “pandemic” that they were healing COVID-19 patients with older drugs such as Ivermectin and hydroxychloroquine.
The annual “flu virus” has all but disappeared since COVID-19 arrived, so this will go down as the biggest medical scam in the history of the human race.
The CDC and FDA acknowledge that there are serious side effects with these vaccines, but they call them “rare,” a term that is really not defined.
Yesterday, however, the FDA announced that they were only recommending the Janssen COVID-19 vaccine now in certain cases, due to the reports of blood clots following the vaccines.
For Immediate Release:
May 05, 2022
Today, the U.S. Food and Drug Administration has limited the authorized use of the Janssen COVID-19 Vaccine to individuals 18 years of age and older for whom other authorized or approved COVID-19 vaccines are not accessible or clinically appropriate, and to individuals 18 years of age and older who elect to receive the Janssen COVID-19 Vaccine because they would otherwise not receive a COVID-19 vaccine.
Today, the U.S. Food and Drug Administration has limited the authorized use of the Janssen COVID-19 Vaccine to individuals 18 years of age and older for whom other authorized or approved COVID-19 vaccines are not accessible or clinically appropriate, and to individuals 18 years of age and older who elect to receive the Janssen COVID-19 Vaccine because they would otherwise not receive a COVID-19 vaccine.
Key Points:
- After conducting an updated analysis, evaluation and investigation of reported cases, the FDA has determined that the risk of thrombosis with thrombocytopenia syndrome (TTS), a syndrome of rare and potentially life-threatening blood clots in combination with low levels of blood platelets with onset of symptoms approximately one to two weeks following administration of the Janssen COVID-19 Vaccine, warrants limiting the authorized use of the vaccine.
- The FDA has determined that the known and potential benefits of the vaccine for the prevention of COVID-19 outweigh the known and potential risks for individuals 18 years of age and older for whom other authorized or approved COVID-19 vaccines are not accessible or clinically appropriate, and for individuals 18 years of age and older who elect to receive the Janssen COVID-19 Vaccine because they would otherwise not receive a COVID-19 vaccine.
- The Fact Sheet for Healthcare Providers Administering Vaccine now reflects the revision of the authorized use of the Janssen COVID-19 Vaccine and includes a warning statement at the beginning of the fact sheet for prominence which summarizes information on the risk for TTS. Additionally, information on the revision to the authorized use of the vaccine and updated information on this risk of blood clots with low levels of blood platelets has been added to the Fact Sheet for Recipients and Caregivers.
Full press release here.

