US Judge Forces FDA to Release Pfizer Covid Vaccine Data – 20,000 Pages by April 1
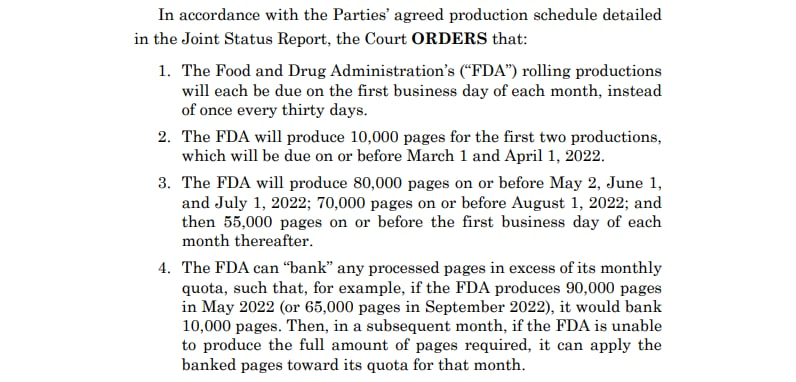
▪️The FDA rolling productions are due on the first business day of every month
▪️10,000 pages for the first two productions are due on or before March 1 and April 1, 2022.
▪️80,000 pages are due before May 2, June 1 and July 1, 2022.
▪️70,000 before August 1, 2022 and then 55K pages first business day each month after.
▪️The FDA can “bank” pages, eg produce excess one month before and deduct on the month after.
Originally, Pfizer and the FDA were planning to release documents a drip at a time over more than half a century.