Pfizer’s vaccine shows dose-related side effects in children; still, the company is letting five-year-olds receive an mRNA dosage that caused serious fevers in slightly younger kids.
Some young children had serious fevers after receiving a medium-sized dose of Pfizer’s mRNA Covid vaccine, a Pfizer clinical trial found.
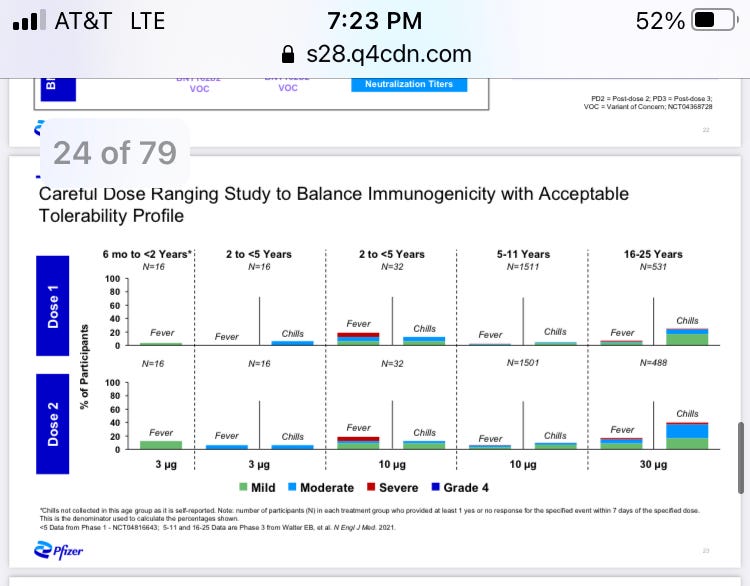
Fevers in kids 2-5 who received 10 micrograms of mRNA were both more common and more severe than those in other children tested with the vaccine, the company found. The side effect occurred after both the first and second vaccine dose.
The company revealed the problems to investors and analysts in mid-December as it disclosed its struggles to produce a Covid vaccine for younger children.
Pfizer ultimately did not move forward with a 10-microgram two-dose regimen for children aged 2 to 5. Instead it is testing a much smaller dose – 3 micrograms – which it had originally intended for kids under 2. That dose did not cause the same problems in early trials.
Pfizer’s larger clinical trial in children under 5 is ongoing. Regulators have not yet authorized Covid vaccines – at any dose – for kids that age.
But they have okayed the vaccine for children from 5 to 12 – at the same 10-microgram level that caused fevers in the clinical trial of younger kids.
The vaccine dosing is not adjusted by weight. All five-year-olds receive the full 10-microgram dose, even if they are no bigger than a typical three or four-year-old.
Pfizer has not published any safety or efficacy data for its vaccine in children under 5 in a peer-reviewed journal, but it did provide a glimpse of the results to investors and analysts on Dec. 17. The data are contained in a single slide in the 79-page presentation.
The red bars represent severe fevers, which clinical trials typically define as higher than 103 degrees.
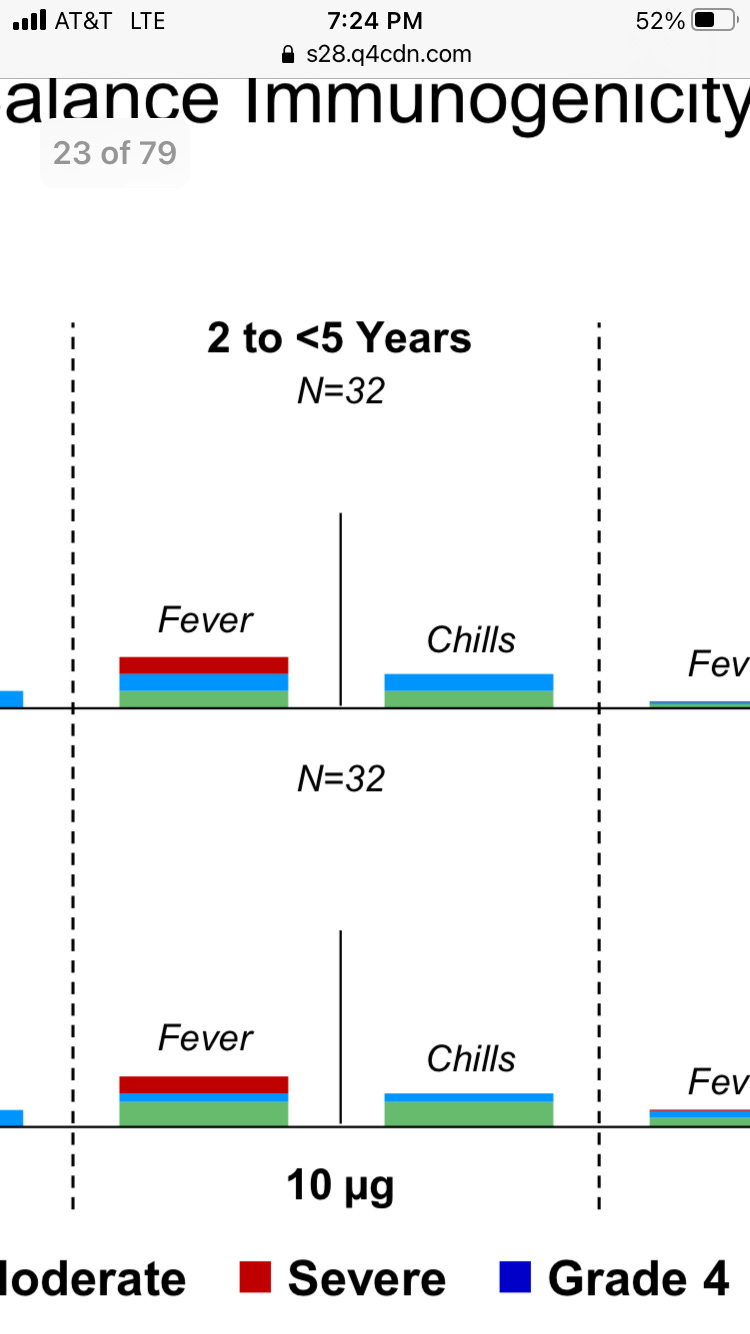
The complete slide is here, showing that roughly 1 in 5 of younger children had fevers after each 10-microgram dose – more than any other children or young adults at any dosage level.
But Pfizer’s hopes for its 3-microgram dose in younger children also appear to be fading.
In the standard two-dose regimen, that dose has proven so ineffective in children 2-5 that the company has now modified its clinical trial to add a third 3 microgram dose.

In adolescents and adults, the Pfizer vaccine dose contains 30 micrograms of mRNA in each shot – three times the dose for kids 5-12 and 10 times the amount that Pfizer is now testing on younger kids. (One million micrograms equal one gram, so the vaccine contains only a tiny amount of material, but it is incredibly biologically potent.)
The company’s struggles with dosing kids under five are part of a bigger issue around vaccinating children.
Pfizer is walking a tightrope as it tries to convince reluctant parents to vaccinate their kids for an illness that is barely a cold for most healthy children.
Despite relentless advertising campaigns and scare stories about children being hospitalized for Covid (stories that frequently reveal about halfway down that the kids were hospitalized for other problems and had incidental Covid diagnoses), only about 1 in 5 American kids 5-12 has received a Covid vaccine.
Pfizer’s own data may only increase that reluctance.

